Study Oversight
The study was supported by National Health Commission of China and designed by the investigators. The study was approved by the institutional review board of the National Health Commission. Written informed consent was waived in light of the urgent need to collect data. Data were analyzed and interpreted by the authors. All the authors reviewed the manuscript and vouch for the accuracy and completeness of the data and for the adherence of the study to the protocol, available with the full text of this article at NEJM.org.
Data Sources
We obtained the medical records and compiled data for hospitalized patients and outpatients with laboratory-confirmed Covid-19, as reported to the National Health Commission between December 11, 2019, and January 29, 2020; the data cutoff for the study was January 31, 2020. Covid-19 was diagnosed on the basis of the WHO interim guidance.14 A confirmed case of Covid-19 was defined as a positive result on high-throughput sequencing or real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of nasal and pharyngeal swab specimens.1 Only laboratory-confirmed cases were included in the analysis.
We obtained data regarding cases outside Hubei province from the National Health Commission. Because of the high workload of clinicians, three outside experts from Guangzhou performed raw data extraction at Wuhan Jinyintan Hospital, where many of the patients with Covid-19 in Wuhan were being treated.
We extracted the recent exposure history, clinical symptoms or signs, and laboratory findings on admission from electronic medical records. Radiologic assessments included chest radiography or computed tomography (CT), and all laboratory testing was performed according to the clinical care needs of the patient. We determined the presence of a radiologic abnormality on the basis of the documentation or description in medical charts; if imaging scans were available, they were reviewed by attending physicians in respiratory medicine who extracted the data. Major disagreement between two reviewers was resolved by consultation with a third reviewer. Laboratory assessments consisted of a complete blood count, blood chemical analysis, coagulation testing, assessment of liver and renal function, and measures of electrolytes, C-reactive protein, procalcitonin, lactate dehydrogenase, and creatine kinase. We defined the degree of severity of Covid-19 (severe vs. nonsevere) at the time of admission using the American Thoracic Society guidelines for community-acquired pneumonia.15
All medical records were copied and sent to the data-processing center in Guangzhou, under the coordination of the National Health Commission. A team of experienced respiratory clinicians reviewed and abstracted the data. Data were entered into a computerized database and cross-checked. If the core data were missing, requests for clarification were sent to the coordinators, who subsequently contacted the attending clinicians.
Study Outcomes
The primary composite end point was admission to an intensive care unit (ICU), the use of mechanical ventilation, or death. These outcomes were used in a previous study to assess the severity of other serious infectious diseases, such as H7N9 infection.16 Secondary end points were the rate of death and the time from symptom onset until the composite end point and until each component of the composite end point.
Study Definitions
The incubation period was defined as the interval between the potential earliest date of contact of the transmission source (wildlife or person with suspected or confirmed case) and the potential earliest date of symptom onset (i.e., cough, fever, fatigue, or myalgia). We excluded incubation periods of less than 1 day because some patients had continuous exposure to contamination sources; in these cases, the latest date of exposure was recorded. The summary statistics of incubation periods were calculated on the basis of 291 patients who had clear information regarding the specific date of exposure.
Fever was defined as an axillary temperature of 37.5°C or higher. Lymphocytopenia was defined as a lymphocyte count of less than 1500 cells per cubic millimeter. Thrombocytopenia was defined as a platelet count of less than 150,000 per cubic millimeter. Additional definitions — including exposure to wildlife, acute respiratory distress syndrome (ARDS), pneumonia, acute kidney failure, acute heart failure, and rhabdomyolysis — are provided in the Supplementary Appendix, available at NEJM.org.
Laboratory Confirmation
Laboratory confirmation of SARS-CoV-2 was performed at the Chinese Center for Disease Prevention and Control before January 23, 2020, and subsequently in certified tertiary care hospitals. RT-PCR assays were performed in accordance with the protocol established by the WHO.17 Details regarding laboratory confirmation processes are provided in the Supplementary Appendix.
Statistical Analysis
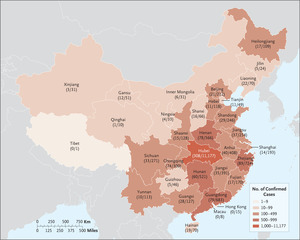
Continuous variables were expressed as medians and interquartile ranges or simple ranges, as appropriate. Categorical variables were summarized as counts and percentages. No imputation was made for missing data. Because the cohort of patients in our study was not derived from random selection, all statistics are deemed to be descriptive only. We used ArcGIS, version 10.2.2, to plot the numbers of patients with reportedly confirmed cases on a map. All the analyses were performed with the use of R software, version 3.6.2 (R Foundation for Statistical Computing).